How To Do An Sds-Page: Tips And Tricks
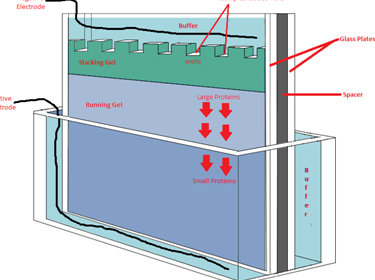
The SDS-PAGE gels are basic and commonly used technique in most research laboratories. Its use is aimed at separating the proteins present in a sample based on their molecular weight. This separation is based on the different speed of protein migration through a polyacrylamide gel when applying an electric field.
In this post we collect some tips and tricks on how to do an SDS-PAGE optimally.
1.- Prepare the reagents in advance . You can do it with all those that are likely to be stored and used for some time without deterioration.
- Buffers: A concentrated solution can be prepared, and a small amount diluted for each experiment.
- Ammonium persulfate: If it is to be used frequently, it can be stored at 4ºC for about a month. For longer periods of time it can be aliquoted and frozen at -20ºC.
- Gels: Gels can also be prepared in advance and stored refrigerated (4ºC) for 10-15 days. In this case it is essential to ensure that they are kept moist until use.
- Samples: They can be prepared and frozen at -20ºC for some months. In this way, once thawed, they will be ready for loading in the gel.
2.- Substitute the distilled water for isopropanol to cover the gel. This will allow the gel to polymerize faster.
3.- Be sure to maintain the proper buffer level in the wells. If the buffer volume drops too low, the wells will dry out and the gel will not run properly.
4.- Prepare the polyacrylamide gel based on the molecular weight of the proteins you are interested in separating. Proteins with higher molecular weights move more slowly through the pores of the gel. The size of these pores can be modulated depending on the size of the proteins to be separated, changing the concentration of polyacrylamide according to the following indicative ranges:
| Molecular weight ofproteins to separate | % polyacrylamide |
| 3-100 kDa | fifteen% |
| 10-200 kDa | 12% |
| 20-300 kDa | 10% |
| 50-500 kDa | 7% |
For protein mixtures with a higher molecular weight range, gradient gels with layers of increasing concentration of polyacrylamide can be used.
5.- If you are running sensitive samples (for example protein-RNA complexes), spray the plates with ethanol (70%) and DEPC water (diethylpyrocarbonate), and make sure they are well dry before starting.
6.- Be careful with the electric power . Although the higher the migration speed of the samples increases, the probability of overheating the gel also increases. To avoid this, consult the manufacturer’s instructions and adjust to the recommended voltage.
7.- High or irregular temperatures throughout the gel directly affect the migration of the samples. There are several ways to keep the temperature low , including filling the outside of the tank with the buffer to the bottom of the wells so that the heat is dispersed evenly throughout the gel.
